Human Tendon-on-a-Chip for Modeling the Myofibroblast Microenvironment in Peritendinous Fibrosis
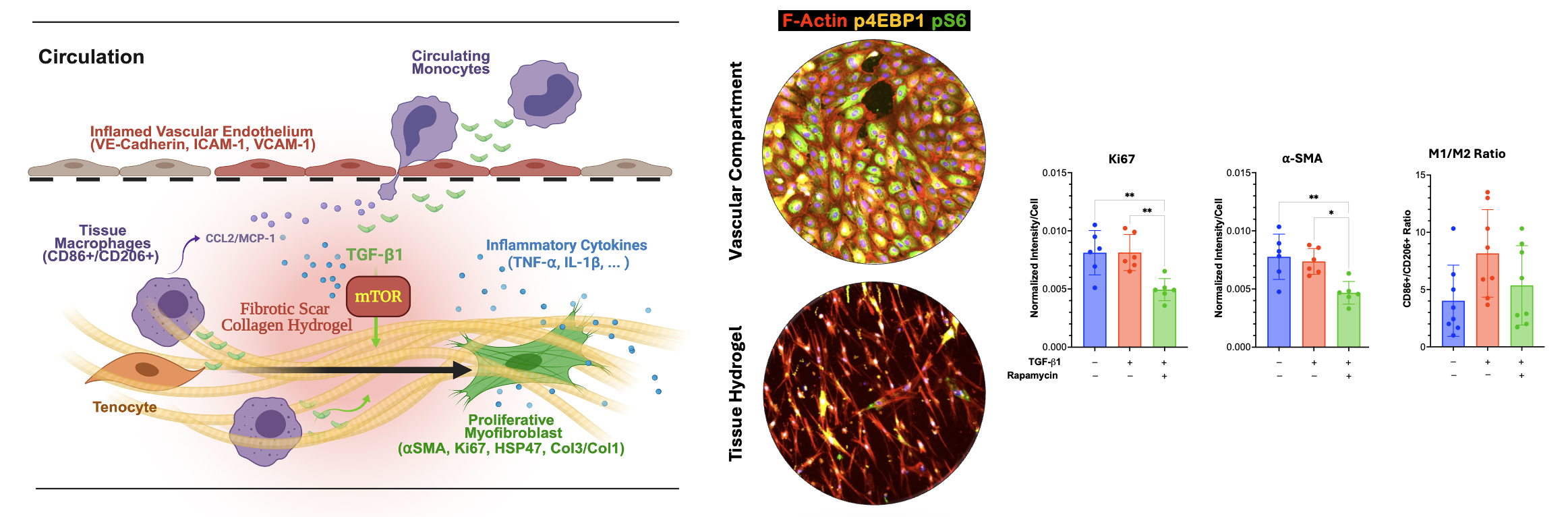
The Human Tendon-on-a-Chip (hToC) platform has emerged from a collaboration between the Awad, McGrath and Miller laboratories at the University of Rochester, supported by a UG3/UH3 (TR003281) award from the National Center for Advancing Translational Sciences and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. These groups share the goal of creating human-relevant in vitro models to dissect the complex cellular crosstalk driving peritendinous fibrosis and the myofibroblast microenvironment. The collaboration launched with the work of Ajalik et al. (2025), who established the hToC as a modular tool capable of simulating the heterotypic interactions between a vascular compartment containing endothelial cells and monocytes and a tissue hydrogel housing tendon fibroblasts and macrophages. By defining the myofibroblast microenvironment, this seminal study demonstrated that the hToC replicates key aspects of in vivo fibrotic phenotypes, including inflammatory cytokine secretion, myofibroblast activation, and matrix contraction driven by TGF-beta1 and macrophage signaling. Transcriptional benchmarking against human tenolysis tissues revealed a shared activation of the mTOR signaling pathway, establishing the platform’s utility for therapeutic screening as demonstrated by the mitigation of fibrotic traits using rapamycin.
Screenshot
Recognizing that the utility of the model relies on replicating the hemodynamic environment of the vasculature, Linares et al. (2025) advanced the platform by developing a manufacturable peel-and-stick flow module that transforms the static hToC into a fluidic system. This work leveraged the modularity of the platform to introduce physiological shear stress, demonstrating that flow significantly enhances endothelial barrier integrity and modulates the expression of adhesion molecules like ICAM-1 in a shear-dependent manner. The integration of flow was shown to create steep chemotactic gradients that robustly drive the multistep cascade of monocyte rolling, arrest, and transmigration, thereby enhancing immune cell infiltration into the tissue compartment compared to static controls. This fluidic capability allows researchers to capture the dynamic interplay between hemodynamic forces and inflammatory signaling that characterizes the early stages of tendon injury.
More recent work has extended the hToC to model the extrinsic mechanisms of adhesion formation between the flexor tendon and the synovial sheath. By incorporating fibroblast-like synoviocytes into a secondary hydrogel adjacent to the tendon tissue, the model recapitulates robust synovial hyperplasia and the formation of physical adhesions bridging the tissue compartments. This configuration identified a novel pathway as a critical driver of this pathology, enabling the successful validation of targeted therapeutics. Integration of photonic sensors is also being done in order to permit real-time analysis of drug response kinetics based on measurement of secreted cytokines.
- Ajalik RE, Linares I, Alenchery RG, Zhang VZ, Wright TW, Miller BL, McGrath JL, Awad HA. Human Tendon-on-a-Chip for Modeling the Myofibroblast Microenvironment in Peritendinous Fibrosis. Adv Healthc Mater. 2025 Feb;14(4):e2403116. doi: 10.1002/adhm.202403116. Epub 2024 Nov 15. PMID: 39544139; PMCID: PMC11804843.
- Linares I, Chen K, Saffren A, Mansouri M, Abhyankar VV, Miller BL, Begolo S, Awad HA, McGrath JL. Fluid flow impacts endothelial-monocyte interactions in a model of vascular inflammatory fibrosis. Sci Rep. 2025 Jan 25;15(1):3227. doi: 10.1038/s41598-025-85987-z. PMID: 39863621; PMCID: PMC11763004.
- Alenchery RG, Ajalik RE, Jerreld K, Midekksa F, Zhong S, Alkatib B, Awad HA. PAI-1 mediates TGF-β1-induced myofibroblast activation in tenocytes via mTOR signaling. J Orthop Res. 2023 Oct;41(10):2163-2174. doi: 10.1002/jor.25594. Epub 2023 May 13. PMID: 37143206; PMCID: PMC10524825.
- Ajalik RE, Alenchery RG, Cognetti JS, Zhang VZ, McGrath JL, Miller BL, Awad HA. Human Organ-on-a-Chip Microphysiological Systems to Model Musculoskeletal Pathologies and Accelerate Therapeutic Discovery. Front Bioeng Biotechnol. 2022 Mar 14;10:846230. doi: 10.3389/fbioe.2022.846230. PMID: 35360391; PMCID: PMC8964284.
- McCloskey MC, Zhang VZ, Ahmad SD, Walker S, Romanick SS, Awad HA, McGrath JL. Sourcing cells for in vitro models of human vascular barriers of inflammation. Front Med Technol. 2022 Oct 21;4:979768. doi: 10.3389/fmedt.2022.979768. PMID: 36483299; PMCID: PMC9724237.
- McCloskey MC, Kasap P, Ahmad SD, Su SH, Chen K, Mansouri M, Ramesh N, Nishihara H, Belyaev Y, Abhyankar VV, Begolo S, Singer BH, Webb KF, Kurabayashi K, Flax J, Waugh RE, Engelhardt B, McGrath JL. The Modular µSiM: A Mass Produced, Rapidly Assembled, and Reconfigurable Platform for the Study of Barrier Tissue Models In Vitro. Adv Healthc Mater. 2022 Sep;11(18):e2200804. doi: 10.1002/adhm.202200804. Epub 2022 Aug 15. PMID: 35899801; PMCID: PMC9580267.
- Cognetti JS, Miller BL. Real-Time, Continuous Monitoring of Tissue Chips as an Emerging Opportunity for Biosensing. Sensors (Basel). 2025 Aug 19;25(16):5153. doi: 10.3390/s25165153. PMID: 40872014; PMCID: PMC12390027.
- Bucukovski J, Miller BL. Everything’s under Control: Maximizing Biosensor Performance through Negative Control Probe Selection. Anal Chem. 2025 Feb 18;97(6):3525-3535. doi: 10.1021/acs.analchem.4c05854. Epub 2025 Feb 3. PMID: 39898999; PMCID: PMC11840803.
