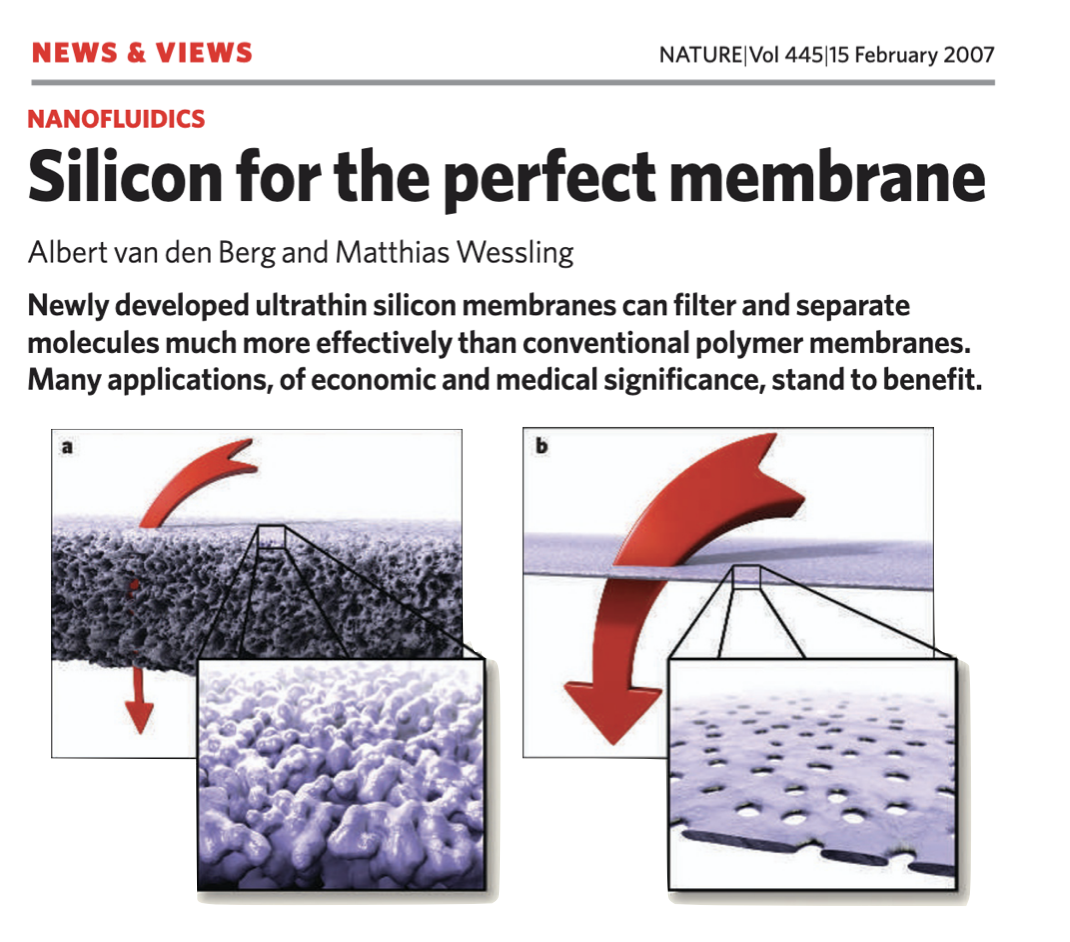
Ultrathin porous silicon ‘nanomembranes’ emerged from the University of Rochester in 2007 with the discovery that rapid thermal annealing of amorphous silicon generates billions of nanometer-scale pores in films only ~15 nm thick1. The freestanding porous membranes were the world’s first porous material in which both the thickness and pore size were on the molecular scale. Described as the ‘perfect membrane,’2 this unique structure predicted transport and sieving properties that are unrivaled by conventional polymer-based filters that are tens to hundreds of microns thick. Record breaking transport and precision separations were confirmed in a decade of subsequent studies on the science of ultrathin membranes3-7, as were other nanomembrane properties including electrical8 and optical9 transparency. In 2014, a more chemically and mechanically robust successor material, nanoporous silicon nitride (NPN), was created by transferring the self-assembled Si-based pore structure into 30 – 100 nm thick silicon nitride (SiN) using the pure nanoporous silicon layer as a sacrificial mask10. This process enabled wafer-scale production at SiMPore Inc. with yields >99%, and the commercial availability and practical use of nanomembranes in devices.
Reliable manufacturing ushered in the “device era” (2013 – 2020) where assembly into various lab-crafted microfluidic devices tested nanomembrane utility in specific applications. Notable examples included composite nanofilter/nanopore sensors for DNA detection11, miniaturized hemodialysis membranes12, and use as electroosmostic microfluidic pumps8. This period also saw the introduction of microporous and microslit membrane products at SiMPore, extending the filter applications of nanomembranes to larger species such as exosomes13, viruses14, nanoparticles15, and bacteria16-18. Optical transparency combines with high membrane permeability and the small (mm2) membrane area to give an unexpected and novel “catch-and-display” utility that simplifies analytic and diagnostic workflows. In this concept, convection through the membrane directs dilute species of interests to pores where they are captured and concentrated in a size-dependent manner. ‘Clogged’ membranes can then be inspected by imaging or spectroscopy to analyze the captured species against a background with little-to-no interference. A dramatic drop in permeability during pore occupancy can even be detected as an increase in hydraulic resistance to create a sensor14. The capture of exosomes in the similarly-sized pores of NPN provides for a highly sensitive ‘digital’ assay of EV function19 and biomarkers20. Using microporous membranes, the same concept has been applied to environmental21, municipal22 and biological23 samples containing microplastics to enable rapid sample processing for confirmation and analysis of this ubiquitous pollutant.
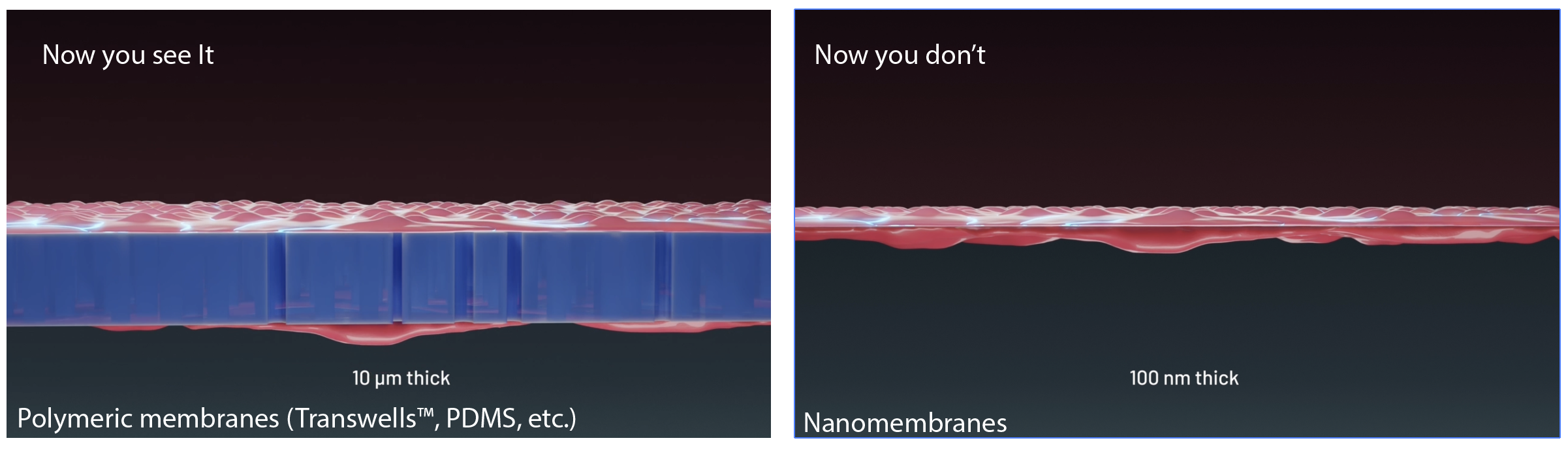
Arguably the most impactful application of nanomembrane technology to date is their use in barrier cell culture and in multicellular models of human “tissue-on-a-chip.” Compatibility with cell culture was established early10 to address an unmet need for porous substrates that overcome the poor imaging characteristics of commercial Transwells™ used to study barrier-forming endothelial and epithelial cells9. Used as a tool for compartmentalizing multicellular tissue models, nanomembranes offer numerous advantages over conventional strategies employing much thicker polymeric membranes or semi-porous walls. Unlike these approaches, nanomembranes provide an ultrathin, exceedingly permeable, and optically clear interface that defines the barrier between tissue compartments. In this way, the exchange of paracrine factors, cytokines, metabolites, and nutrients occurs at rates set by the cells and their self-assembled matrices rather than by the membrane itself. Because the membranes are thinner than the wavelength of light and thinner than a confocal optical slice, they are undetectable in fluorescence microscopy and vanish in 3D reconstructions of the basement membranes formed at compartment interfaces24,25. Innovative, dual-scale nanomembranes support controlled transcellular transmigration and multicellular interactions through patterned micropores while preserving the diffusive continuity of the nanoporous background26. Democratizing access to the unique qualities of nanomembranes for barrier and tissue-chip development is the primary motivation of µSiM27: a modular microfluidic device that enables non-engineering laboratories to design, build and manufacture custom chips for diverse tissue models17,24,28-30.
- Striemer, C. C., Gaborski, T. R., McGrath, J. L. & Fauchet, P. M. Charge- and size-based separation of macromolecules using ultrathin silicon membranes. Nature 445, 749-753 (2007). https://doi.org/10.1038/nature05532
- van den Berg, A. & Wessling, M. Silicon for the perfect membrane. Nature 445, 726 (2007).
- Lucas, K., Ahmad, S. D., Dehghani, M., Gaborski, T. & McGrath, J. Critical flux behavior of ultrathin membranes in protein-rich solutions. Separation and Purification Technology 251 (2020). https://doi.org/10.1016/j.seppur.2020.117342
- Smith, K. J. P., May, M., Baltus, R. & McGrath, J. L. A predictive model of separations in dead-end filtration with ultrathin membranes. Separation and Purification Technology 189, 40-47 (2017). https://doi.org/10.1016/j.seppur.2017.07.032
- Snyder, J. L., Clark, A., Jr., Fang, D. Z., Gaborski, T. R., Striemer, C. C., Fauchet, P. M. & McGrath, J. L. An experimental and theoretical analysis of molecular separations by diffusion through ultrathin nanoporous membranes. J Memb Sci 369, 119-129 (2011). https://doi.org/10.1016/j.memsci.2010.11.056
- Winans, J. D., Smith, K. J. P., Gaborski, T. R., Roussie, J. A. & McGrath, J. L. Membrane capacity and fouling mechanisms for ultrathin nanomembranes in dead-end filtration. Journal of Membrane Science 499, 282-289 (2016). https://doi.org/10.1016/j.memsci.2015.10.053
- Gaborski, T. R., Snyder, J. L., Striemer, C. C., Fang, D. Z., Hoffman, M., Fauchet, P. M. & McGrath, J. L. High-performance separation of nanoparticles with ultrathin porous nanocrystalline silicon membranes. ACS Nano 4, 6973-6981 (2010). https://doi.org/10.1021/nn102064c
- Snyder, J. L., Getpreecharsawas, J., Fang, D. Z., Gaborski, T. R., Striemer, C. C., Fauchet, P. M., Borkholder, D. A. & McGrath, J. L. High-performance, low-voltage electroosmotic pumps with molecularly thin silicon nanomembranes. Proc Natl Acad Sci U S A 110, 18425-18430 (2013). https://doi.org/10.1073/pnas.1308109110
- Carter, R. N., Casillo, S. M., Mazzocchi, A. R., DesOrmeaux, J. S., Roussie, J. A. & Gaborski, T. R. Ultrathin transparent membranes for cellular barrier and co-culture models. Biofabrication 9, 015019 (2017). https://doi.org/10.1088/1758-5090/aa5ba7
- DesOrmeaux, J. P. S., Winans, J. D., Wayson, S. E., Gaborski, T. R., Khire, T. S., Striemer, C. C. & McGrath, J. L. Nanoporous silicon nitride membranes fabricated from porous nanocrystalline silicon templates. Nanoscale 6, 10798-10805 (2014). https://doi.org/Doi 10.1039/C4nr03070b
- Briggs, K., Madejski, G., Magill, M., Kastritis, K., de Haan, H. W., McGrath, J. L. & Tabard-Cossa, V. DNA Translocations through Nanopores under Nanoscale Preconfinement. Nano Lett 18, 660-668 (2018). https://doi.org/10.1021/acs.nanolett.7b03987
- Hill, K., Walker, S. N., Salminen, A., Chung, H. L., Li, X., Ezzat, B., Miller, J. J., DesOrmeaux, J. S., Zhang, J., Hayden, A., Burgin, T., Piraino, L., May, M. N., Gaborski, T. R., Roussie, J. A., Taylor, J., DiVincenti, L., Jr., Shestopalov, A. A., McGrath, J. L. & Johnson, D. G. Second Generation Nanoporous Silicon Nitride Membranes for High Toxin Clearance and Small Format Hemodialysis. Adv Healthc Mater 9, e1900750 (2020). https://doi.org/10.1002/adhm.201900750
- Dehghani, M., Lucas, K., Flax, J., McGrath, J. & Gaborski, T. Tangential flow microfluidics for the capture and release of nanoparticles and extracellular vesicles on conventional and ultrathin membranes. Adv Mater Technol 4 (2019). https://doi.org/10.1002/admt.201900539
- Klaczko, M. E., Lucas, K., Salminen, A. T., McCloskey, M. C., Ozgurun, B., Ward, B. M., Flax, J. & McGrath, J. L. Rapid and specific detection of intact viral particles using functionalized microslit silicon membranes as a fouling-based sensor. Analyst 147, 213-222 (2022). https://doi.org/10.1039/d1an01504d
- Lucas, K., Dehghani, M., Khire, T., Gaborski, T., Flax, J. D., Waugh, R. E. & McGrath, J. L. A predictive model of nanoparticle capture on ultrathin nanoporous membranes. Journal of Membrane Science 633 (2021). https://doi.org/10.1016/j.memsci.2021.119357
- de Mesy Bentley, K. L., Trombetta, R., Nishitani, K., Bello-Irizarry, S. N., Ninomiya, M., Zhang, L., Chung, H. L., McGrath, J. L., Daiss, J. L., Awad, H. A., Kates, S. L. & Schwarz, E. M. Evidence of Staphylococcus Aureus Deformation, Proliferation, and Migration in Canaliculi of Live Cortical Bone in Murine Models of Osteomyelitis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 32, 985-990 (2017). https://doi.org/10.1002/jbmr.3055
- Masters, E. A., Salminen, A. T., Begolo, S., Luke, E. N., Barrett, S. C., Overby, C. T., Gill, A. L., de Mesy Bentley, K. L., Awad, H. A., Gill, S. R., Schwarz, E. M. & McGrath, J. L. An in vitro platform for elucidating the molecular genetics of S. aureus invasion of the osteocyte lacuno-canalicular network during chronic osteomyelitis. Nanomedicine 21, 102039 (2019). https://doi.org/10.1016/j.nano.2019.102039
- Wright, E., Miller, J. J., Csordas, M., Gosselin, A. R., Carter, J. A., McGrath, J. L., Latulippe, D. R. & Roussie, J. A. Development of isoporous microslit silicon nitride membranes for sterile filtration applications. Biotechnol Bioeng 117, 879-885 (2020). https://doi.org/10.1002/bit.27240
- Riazanski, V., Mauleon, G., Lucas, K., Walker, S., Zimnicka, A. M., McGrath, J. L. & Nelson, D. J. Real time imaging of single extracellular vesicle pH regulation in a microfluidic cross-flow filtration platform. Commun Biol 5, 13 (2022). https://doi.org/10.1038/s42003-021-02965-7
- Walker, S. N., Lucas, K., Dewey, M. J., Badylak, S. F., Hussey, G. S., Flax, J. & McGrath, J. L. Rapid Assessment of Biomarkers on Single Extracellular Vesicles Using “Catch and Display” on Ultrathin Nanoporous Silicon Nitride Membranes. Small 21, 2405505 (2025). https://doi.org/https://doi.org/10.1002/smll.202405505
- Romanick, S. S., Madejski, G., Cashion, G., Berger, A. J., Elder, A. & McGrath, J. Assessment of household settled dust via silicon nanomembrane analysis pipeline (SNAP). Environmental Technology & Innovation 38 (2025). https://doi.org/10.1016/j.eti.2025.104106
- Madejski, G. R., Ahmad, S. D., Musgrave, J., Flax, J., Madejski, J. G., Rowley, D. A., DeLouise, L. A., Berger, A. J., Knox, W. H. & McGrath, J. L. Silicon Nanomembrane Filtration and Imaging for the Evaluation of Microplastic Entrainment along a Municipal Water Delivery Route. Sustainability 12 (2020). https://doi.org/10.3390/su122410655
- Cai, B., De Jesus Andino, F., McGrath, J. L., Romanick, S. S. & Robert, J. Ingestion of polyethylene terephthalate microplastic water contaminants by Xenopus laevis tadpoles negatively affects their resistance to ranavirus infection and antiviral immunity. Environ Pollut 356, 124340 (2024). https://doi.org/10.1016/j.envpol.2024.124340
- McCloskey, M. C., Ahmad, S. D., Widom, L. P., Kasap, P., Gastfriend, B. D., Shusta, E. V., Palecek, S. P., Engelhardt, B., Gaborski, T. R., Flax, J., Waugh, R. E. & McGrath, J. Pericytes enrich the basement membrane and reduce neutrophil transmigration in an in vitro model of peripheral inflammation at the blood brain barrier. Biomaterials Research 28, 0081 (2024). https://doi.org/10.34133/bmr.0081
- Trempel, M. A., Du, Y., Widom, L. P., Reitz, E. E., Feidler, A. M., Kasap, P., Engelhardt, B., Gaborski, T. R., Gelbard, H. A., Terrando, N. & McGrath, J. L. Pericytes repair engineered defects in the basement membrane to restore barrier integrity in an in vitro model of the blood-brain barrier. Mater Today Bio 35, 102361 (2025). https://doi.org/10.1016/j.mtbio.2025.102361
- Salminen, A. T., Zhang, J., Madejski, G. R., Khire, T. S., Waugh, R. E., McGrath, J. L. & Gaborski, T. R. Ultrathin Dual-Scale Nano- and Microporous Membranes for Vascular Transmigration Models. Small 15, e1804111 (2019). https://doi.org/10.1002/smll.201804111
- McCloskey, M. C., Kasap, P., Ahmad, S. D., Su, S. H., Chen, K., Mansouri, M., Ramesh, N., Nishihara, H., Belyaev, Y., Abhyankar, V. V., Begolo, S., Singer, B. H., Webb, K. F., Kurabayashi, K., Flax, J., Waugh, R. E., Engelhardt, B. & McGrath, J. L. The Modular microSiM: A Mass Produced, Rapidly Assembled, and Reconfigurable Platform for the Study of Barrier Tissue Models In Vitro. Adv Healthc Mater 11, e2200804 (2022). https://doi.org/10.1002/adhm.202200804
- Ahmad, D., Linares, I., Pietropaoli, A., Waugh, R. E. & McGrath, J. L. Sided Stimulation of Endothelial Cells Modulates Neutrophil Trafficking in an In Vitro Sepsis Model. Adv Healthc Mater, e2304338 (2024). https://doi.org/10.1002/adhm.202304338
- Ajalik, R. E., Linares, I., Alenchery, R. G., Zhang, V. Z., Wright, T. W., Miller, B. L., McGrath, J. L. & Awad, H. A. Human Tendon-on-a-Chip for Modeling the Myofibroblast Microenvironment in Peritendinous Fibrosis. Adv Healthc Mater 14, e2403116 (2025). https://doi.org/10.1002/adhm.202403116
- Chen, K., Linares, I. M., Trempel, M. A., Feidler, A. M., De Silva, D., Farajollahi, S., Jones, J., Kuebel, J., Kasap, P., Engelhardt, B., Flax, J., Abhyankar, V. V., Waugh, R. E., Gelbard, H. A., Terrando, N. & McGrath, J. L. Shear Conditioning Promotes Microvascular Endothelial Barrier Resilience in a Human BBB-on-a-Chip Model of Systemic Inflammation Leading to Astrogliosis. Adv Sci (Weinh), e08271 (2025). https://doi.org/10.1002/advs.202508271