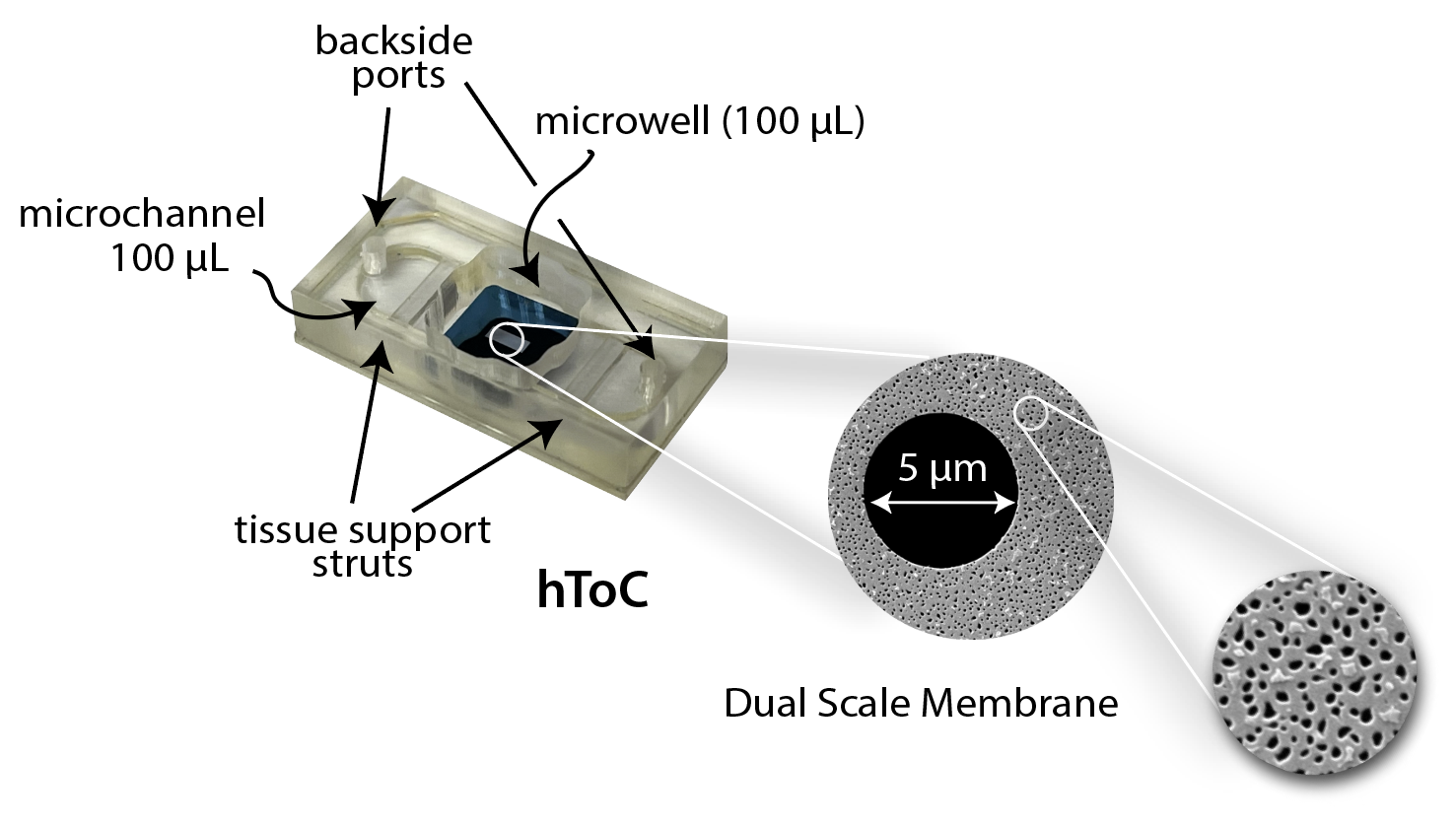
The human Tissue-on-a-Chip (hToC) is a platform that facilitates the culture of a vascular barriers directly adjacent to a 3D human tissue model1,2. As in the µSiM3, a key enabling element is an ultrathin (<100 nm), optically invisible, and highly permeable silicon nitride membrane4,5. More specifically, the hToC features dual-scale nanomembranes that combine nanopores with thousands of patterned micropores6. The nanoporous background provides unhindered diffusion of paracrine factors, while the micropores support cell trafficking from one compartment to the other and enable direct cell-cell contact between tissue and barrier cells7. The top component of the hToC chip features a 100 µL open well and ports to access the bottom component fluidically. This component is identical to the µSiM top component. The bottom component features rigid tissue-support bars that are suspended laterally across a 100 µL microchannel. These struts are another enabling feature of the hToC: They enable the culture of fibroblasts or other other contractile cells in an extended hydrogel while preventing gel detachment and collapse as cells as cells remodel matrix and exert tension.
 In a major innovation in MPS platform design, hToC workflows can leverage the modular, two-component design to allow the barrier and 3D tissue compartments to be cultured independently under their own optimal conditions before being brought together1. Some vascular barriers only take days to mature, while stromal or other 3D tissues may require a week or more to establish matrix organization, cellular polarization, and/or macrophage differentiation. By culturing each compartment separately, the hToC accommodates these distinct developmental timelines, improves experimental reproducibility, and enables precise control of when and how the two systems interact. Assembly is performed in minutes using pressure-sensitive adhesives, creating a sealed, imaging-ready barrier–tissue interface without perturbing either culture. If desired, the device can later be disassembled non-destructively to perform compartment-specific analyses such as transcriptomics, immunostaining, cytokine profiling from each side of the interface. This modularity enhances experimental flexibility and supports a broad range of biological questions, including barrier-initiated signaling, tissue-driven inflammation, and the dynamic interplay between vascular and tissue compartments.
In a major innovation in MPS platform design, hToC workflows can leverage the modular, two-component design to allow the barrier and 3D tissue compartments to be cultured independently under their own optimal conditions before being brought together1. Some vascular barriers only take days to mature, while stromal or other 3D tissues may require a week or more to establish matrix organization, cellular polarization, and/or macrophage differentiation. By culturing each compartment separately, the hToC accommodates these distinct developmental timelines, improves experimental reproducibility, and enables precise control of when and how the two systems interact. Assembly is performed in minutes using pressure-sensitive adhesives, creating a sealed, imaging-ready barrier–tissue interface without perturbing either culture. If desired, the device can later be disassembled non-destructively to perform compartment-specific analyses such as transcriptomics, immunostaining, cytokine profiling from each side of the interface. This modularity enhances experimental flexibility and supports a broad range of biological questions, including barrier-initiated signaling, tissue-driven inflammation, and the dynamic interplay between vascular and tissue compartments.
- Ajalik RE, Linares I, Alenchery RG, Zhang VZ, Wright TW, Miller BL, McGrath JL, Awad HA. Human Tendon-on-a-Chip for Modeling the Myofibroblast Microenvironment in Peritendinous Fibrosis. Adv Healthc Mater. 2025;14(4):e2403116.
- Linares I, Chen K, Saffren A, Mansouri M, Abhyankar VV, Miller BL, Begolo S, Awad HA, McGrath JL. Fluid flow impacts endothelial-monocyte interactions in a model of vascular inflammatory fibrosis. Scientific Reports. 2025;15(1):3227.
- McCloskey MC, Kasap P, Ahmad SD, Su SH, Chen K, Mansouri M, Ramesh N, Nishihara H, Belyaev Y, Abhyankar VV, Begolo S, Singer BH, Webb KF, Kurabayashi K, Flax J, Waugh RE, Engelhardt B, McGrath JL. The Modular muSiM: a Mass Produced, Rapidly Assembled, and Reconfigurable Platform for the Study of Barrier Tissue Models In Vitro. Adv Healthc Mater. 2022:e2200804.
- DesOrmeaux JPS, Winans JD, Wayson SE, Gaborski TR, Khire TS, Striemer CC, McGrath JL. Nanoporous silicon nitride membranes fabricated from porous nanocrystalline silicon templates. Nanoscale. 2014;6(18):10798-805.
- Striemer CC, Fauchet PM, Gaborski TR, McGrath JL, inventors; University of Rochester, assignee. Ultrathin Porous Nanoscale Membranes, Methods of Making, and Uses Thereof patent 8,518,276.
- Salminen AT, Zhang J, Madejski GR, Khire TS, Waugh RE, McGrath JL, Gaborski TR. Ultrathin Dual-Scale Nano- and Microporous Membranes for Vascular Transmigration Models. Small. 2019:15:e1804111.
- Trempel MA, Du Y, Widom LP, Reitz EE, Feidler AM, Kasap P, Engelhardt B, Gaborski TR, Gelbard HA, Terrando N, McGrath JL. Pericytes repair engineered defects in the basement membrane to restore barrier integrity in an in vitro model of the blood-brain barrier. Mater Today Bio. 2025;35:102361.
- Wright E, Miller JJ, Csordas M, Gosselin AR, Carter JA, McGrath JL, Latulippe DR, Roussie JA. Development of isoporous microslit silicon nitride membranes for sterile filtration applications. Biotechnol Bioeng. 2019;117:879-85.
- Salminen AT, McCloskey MC, Ahmad SD, Romanick SS, Chen K, Houlihan W, Klaczko ME, Flax J, Waugh RE, McGrath JL. Molecular mechanisms underlying the heterogeneous barrier responses of two primary endothelial cell types to sphingosine-1-phosphate. Eur J Cell Biol. 2022;101(3):151233.
