The µSiM-BBB model has evolved through a long-standing collaboration between the McGrath (Biomedical Engineering; URochester) and Engelhardt (Neuroimmunology; UBern) laboratories. These laboratories share the goal of creating human-relevant in vitro models to study the role of circulating immune cells in CNS health and disease. The collaboration launched with work of Mossu et al.1, who first used the ultrathin silicon-nitride nanomembranes in a hand-crafted pre-cursor to the µSiM device. This seminal work demonstrated image T-cell arrest, crawling, and diapedesis across human brain microvascular endothelial barriers, visualizing this transmigration with a clarity not possible on conventional membranes. The paper established nanomembranes as a uniquely powerful tool for mechanistic analysis of immune–BBB interactions and defined core value propositions for the µSiM: glass-like optical transparency to enable high quality live, label-free, and fluorescence imaging on a permeable substrate; excellent barrier formation; and the successful integration of microfluidics for the introduction of immune cells under flow. The Engelhardt lab has continued application of nanomembrane-based BBB models to gain mechanistic insights about T-cell diapedesis pathways and junctional biology, including the notable identification of tricellular junctions as the preferred site of leukocyte entry2,3.
Recognizing that the utility of nanomembranes for BBB and other barrier studies would only be realized if non-engineering laboratories could quickly and independently access the tool became the major impetus for the modular µSiM4—a manufactured yet flexible platform designed to make reproducible nanomembrane-based cell culture broadly available. McCloskey et al.4 introduced the modular µSiM design as a novel snap-together chip that that could be assembled from mass-produced components without microfabrication expertise. The paper also introduced the use of hiPSC-derived brain microvascular endothelial cells and validated novel µSiM-specific assays for permeability, inflammatory stimulation and response, imaging, and immune cell transmigration, with reproducibility demonstrated between Rochester and Bern. In a companion study, Mansouri et al5 introduced a reconfigurable flow module for the modular µSiM that adds microfluidic shear conditioning and perfusion on demand, allowing users to culture cells with standard open-well protocols and then switch to physiologic flow to study shear-dependent endothelial responses and immune-cell transmigration. A follow-up study from the University of Michigan further leveraged the modularity of the µSiM format by interfacing the device with a particle-based digital ELISA plate, enabling longitudinal, apical-to-basal profiling of cytokine secretion from brain microvascular endothelial cells following an inflammatory challenge.6
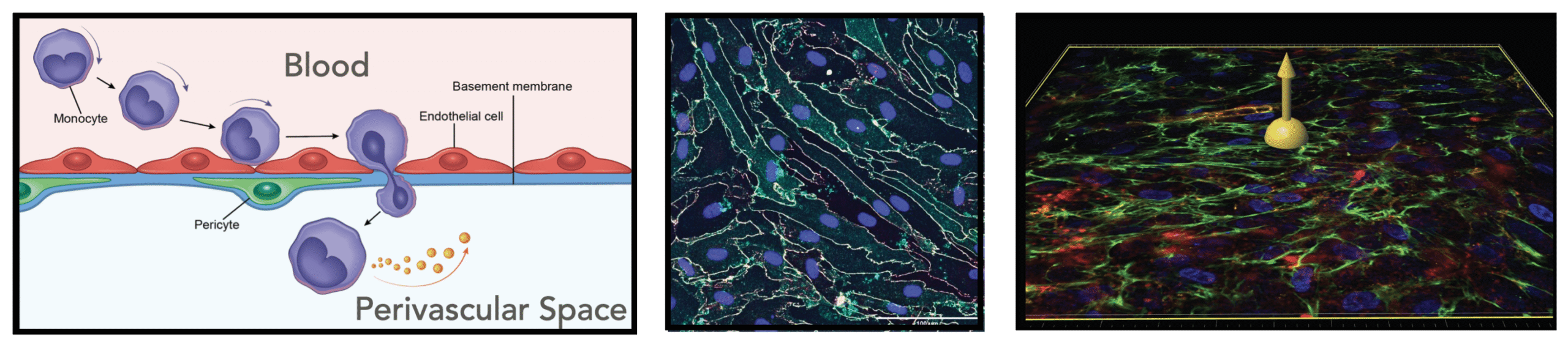
More recent work has extended the µSiM-based BBB to incorporate higher-order cellular, structural, and functional features, increasing both its physiological relevance and its utility for probing disease mechanisms. McCloskey, Ahmad, et al.7 used isogenically matched hiPSC-derived pericyte-like cells to demonstrate that pericytes enrich the abluminal basement membrane and selectively regulate neutrophil transmigration under sepsis-like cytokine stimulation, establishing a human reductionist model for pericyte-dependent inflammatory gating at the BBB. Trempel et al.8 further revealed that pericytes not only stabilize barrier function but actively repair engineered defects in the basement membrane – restoring laminin continuity, preventing BMEC transmigration through large micropores, and modeling a fundamental structural maintenance role previously inaccessible in vitro. In parallel, Chen et al.9 introduced microfluidic shear conditioning to the BBB on the µSiM for the first time, demonstrating both the anti-inflammatory effects of physiologic flow on endothelial activation and the successful transduction of a circulating inflammatory challenge into a canonical hallmark of CNS injury, astrocyte activation reminiscent of astrogliosis.
- Mossu, A., Rosito, M., Khire, T., Li Chung, H., Nishihara, H., Gruber, I., Luke, E., Dehouck, L., Sallusto, F., Gosselet, F., McGrath, J. L. & Engelhardt, B. A silicon nanomembrane platform for the visualization of immune cell trafficking across the human blood-brain barrier under flow. J Cereb Blood Flow Metab 39, 395-410 (2019). https://doi.org/10.1177/0271678X18820584
- Castro Dias, M., Odriozola Quesada, A., Soldati, S., Bosch, F., Gruber, I., Hildbrand, T., Sonmez, D., Khire, T., Witz, G., McGrath, J. L., Piontek, J., Kondoh, M., Deutsch, U., Zuber, B. & Engelhardt, B. Brain endothelial tricellular junctions as novel sites for T cell diapedesis across the blood-brain barrier. J Cell Sci 134 (2021). https://doi.org/10.1242/jcs.253880
- Soldati, S., Bar, A., Vladymyrov, M., Glavin, D., McGrath, J. L., Gosselet, F., Nishihara, H., Goelz, S. & Engelhardt, B. High levels of endothelial ICAM-1 prohibit natalizumab mediated abrogation of CD4(+) T cell arrest on the inflamed BBB under flow in vitro. J Neuroinflammation 20, 123 (2023). https://doi.org/10.1186/s12974-023-02797-8
- McCloskey, M. C., Kasap, P., Ahmad, S. D., Su, S. H., Chen, K., Mansouri, M., Ramesh, N., Nishihara, H., Belyaev, Y., Abhyankar, V. V., Begolo, S., Singer, B. H., Webb, K. F., Kurabayashi, K., Flax, J., Waugh, R. E., Engelhardt, B. & McGrath, J. L. The Modular microSiM: A Mass Produced, Rapidly Assembled, and Reconfigurable Platform for the Study of Barrier Tissue Models In Vitro. Adv Healthc Mater 11, e2200804 (2022). https://doi.org/10.1002/adhm.202200804
- Mansouri, M., Ahmed, A., Ahmad, S. D., McCloskey, M. C., Joshi, I. M., Gaborski, T. R., Waugh, R. E., McGrath, J. L., Day, S. W. & Abhyankar, V. V. The Modular microSiM Reconfigured: Integration of Microfluidic Capabilities to Study In Vitro Barrier Tissue Models under Flow. Adv Healthc Mater 11, e2200802 (2022). https://doi.org/10.1002/adhm.202200802
- Su, S.-H., Song, Y., Stephens, A., Situ, M., McCloskey, M. C., McGrath, J. L., Andjelkovic, A. V., Singer, B. H. & Kurabayashi, K. A tissue chip with integrated digital immunosensors: In situ brain endothelial barrier cytokine secretion monitoring. Biosensors and Bioelectronics 224 (2023). https://doi.org/10.1016/j.bios.2022.115030
- McCloskey, M. C., Ahmad, S. D., Widom, L. P., Kasap, P., Gastfriend, B. D., Shusta, E. V., Palecek, S. P., Engelhardt, B., Gaborski, T. R., Flax, J., Waugh, R. E. & McGrath, J. Pericytes enrich the basement membrane and reduce neutrophil transmigration in an in vitro model of peripheral inflammation at the blood brain barrier. Biomaterials Research 28, 0081 (2024). https://doi.org/10.34133/bmr.0081
- Trempel, M. A., Du, Y., Widom, L. P., Reitz, E. E., Feidler, A. M., Kasap, P., Engelhardt, B., Gaborski, T. R., Gelbard, H. A., Terrando, N. & McGrath, J. L. Pericytes repair engineered defects in the basement membrane to restore barrier integrity in an in vitro model of the blood-brain barrier. Mater Today Bio 35, 102361 (2025). https://doi.org/10.1016/j.mtbio.2025.102361
- Chen, K., Linares, I. M., Trempel, M. A., Feidler, A. M., De Silva, D., Farajollahi, S., Jones, J., Kuebel, J., Kasap, P., Engelhardt, B., Flax, J., Abhyankar, V. V., Waugh, R. E., Gelbard, H. A., Terrando, N. & McGrath, J. L. Shear Conditioning Promotes Microvascular Endothelial Barrier Resilience in a Human BBB-on-a-Chip Model of Systemic Inflammation Leading to Astrogliosis. Adv Sci (Weinh), e08271 (2025). https://doi.org/10.1002/advs.202508271
